# Review of Fundus Histology and Physiology
First of all, what’s all this about a fundus? I thought that was part of the stomach!?!?
To an ophthalmologist, the fundus is the inside of the back of the eyeball. Evaluation of the fundus includes evaluation of the retina, choroid and sclera.
Be sure to thoroughly review the retinal portion of your anatomy lab session from earlier in the term.
Retina – There are about 60 cell types in the retina, but the most important are the retinal pigment epithelium (RPE), photoreceptors (rods and cones), bipolar cells, and ganglion cells. Neural transmission (labelled “signal transduction” in the cartoon below) starts in the photoreceptor and proceeds to the bipolar then to the ganglion cells. Therefore the “neurosensory retina” is everything minus the RPE. Rhodopsin (a combination of retinaldehyde and the protein opsin) is the photopigment in the rods that binds to photons of light. Iodopsin is the photopigment in cones. The optic nerve is collection of ganglion cell axons that travel to the brain. Rod cells respond to dim light and are particularly sensitive to movement in the visual environment. Cone cells have differential “spectral sensitivities” that impart color vision, and they only respond to bright light
Choroid - between the retina and sclera. Contains the tapetum (if one is present), heavy pigmentation in most individuals (although nonpigmented choroidal tissue is a normal variation), and many vessels. The choroidal vasculature has three relatively distinct layers of vessels: a large vessel layer posteriorly, a layer of somewhat smaller vessels anterior to the large vessel layer, and a capillary layer (called the choriocapillaris) anterior to that. The choroid is part of the uveal tract, and like the entire uveal tract it is highly vascular. In the dog (in many species, in fact) the choroid provides a great deal of the nutrition to the retina.
Sclera - exterior to the choroid. Relatively acellular, avascular, and essentially nonpigmented.
# Evaluation of the Retina and Optic Nerve
Techniques used in examination of the retina
Principal exam technique is ophthalmoscopy. This may include direct and indirect ophthalmoscopy, with the indirect technique being the preferred method (you need to review advantages / disadvantages of indirect vs. direct ophthalmoscopy.
Indirect ophthalmoscopy should be done in a systematic fashion to avoid overlooking lesions because you forgot to look for them:
Optic disc – evaluate for:
Size and shape, which are highly variable in dogs
Color - normal is a whitish-salmon pink color
Is the disc in the same plane as the retina, or is it depressed or elevated?
Physiologic pit - a small central depression representing the caudal course of the ganglion cell axons as they head caudally towards the brain.
The feline disc is not myelinated, and is therefore round and darker.
Vessels
Number of vessels - there is generally a triad or tetrad of major vein-artery pairs, with a number of smaller arterioles and venules between them. Veins usually extend to the center of the disc (where they often join in an anastomotic arc or circle), while arterioles do not.
Caliber or width of vessels. The veins have a larger diameter than the arteries.
Number of vascular crossings….if you’re not seeing many crossings, it’s probably due to a decrease in the number of vessels.
Degree of branching of the vessels
Do the vessels go all the way to the periphery?
Tapetum
Please be aware that the tapetum is actually a choroidal structure, not a retinal structure.
Homogeneity - If the tapetum is the same everywhere, it is probably normal for that animal. The absence of a tapetum in a species that usually has a tapetum is considered a normal variation - this is most often seen in “color dilute” individuals (e.g., Siberian huskies, merle collies).
Nontapetum
As with the tapetum, most of what you’ll see in the nontapetum (which is typically going to be dark brown pigment, and sometimes choroidal vessels) is in the choroid, not the retina.
Homogeneity - again, if it’s the same everywhere it’s probably normal.
Depigmented lesions - usually indicate retinal scarring. But be aware that there is a lot of individual variation in the amount of nontapetal pigment, which exposes the underlying choroidal vasculature.
Pupillary light responses - direct and indirect
Menace response
Tracking (cotton balls)/Maze test
Dazzle response
Electroretinogram (ERG)
Measures electrical activity in the retina in response to a flash of light.
By using various combinations of dark adaptation and colored filters, can separate rod and core contributions of the ERG.
Only the photoreceptors and dipolar cells contribute to the ERG; the ERG can be normal even if the optic nerve is transected.
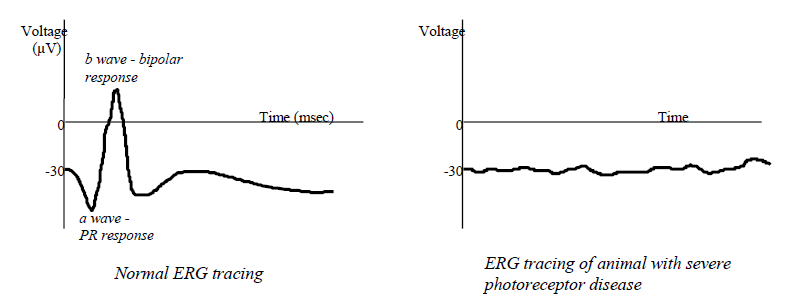
If a dog is blind and the ERG is normal, a CT or MRI is usually indicated as the disease is caudal to the retina.
Visual Evoked Potential (VEP)
Measures electrical activity in the visual cortex in response to a flash of light.
Photoreceptors, bipolar cells, ganglion cells, optic nerve, and visual cortex must all be intact to have a normal VEP
# Retinal Diseases
# Developmental Abnormalities
# Collie Eye Anomaly
Definition: Inherited, congenital malformation of fundus of Collies. Primary defect is one of maldifferentiation of the neural crest cells (your text inaccurately says “mesodermal”) in the posterior pole of the eye resulting in lesions described below.
Clinical signs/history:
- Usually no signs or significant history. A small number of these animals are blind, and may therefore present with that complaint. Most are discovered incidentally, or when owners familiar with the syndrome ask you specifically to look for it.
Ophthalmoscopic appearance:
Focal area of choriodal hypoplasia temporal to the optic disc. BASIC LESION OF THIS SYNDROME! Vision unimpaired
Optic disc coloboma. Vision usually unimpaired
Retinal detachment. Blind.
Intraocular hemorrhage. Blind.
These lesions build on one another; i.e., can’t have coloboma without hypoplasia, etc.
Etiology:
- Inherited; autosomal recessive. Multiple phenotypes, but only one genotype!!
Other general features of this syndrome:
Estimates put number of affected dogs in US at 70 - 90%.
Most dogs affected with hypoplasia. Only about 5% have blinding manifestations – thus the perpetuation of the myth that the condition “breeds true” phenotypically.
Nonprogressive, but beware of “Go-normals”
Other Collie-ish breeds are affected (Sheltie, Border Collie, Aussie, G. Shepherd), but at nowhere near the frequency of Collies.
Treatment
- None. Counsel owner as to maintenance of a blind animal if, in fact, animal is blind. Do not breed, regardless of the manifestation.
# Merle Ocular Dysgenesis
Red and Blue Australian Shepherds
Clinical signs
Microphthalmia
Heterochromia, dyscoria, corectopia
Cataracts
Large equatorial staphylomas (i.e., outpouchings of sclera)
Retinal detachments
Etiology
Inherited: autosomal recessive, incomplete penetrance
Affected animals are homozygous for the merling gene and are excessively white
# Retinal Dysplasia
Definition: Congenital maldevelopment and improper differentiation of the neurosensory retina. Can be either inherited or due to various in utero insults during the period of retinal differentiation.
Variations and Breeds
There is a tremendous amount of controversy about the categorization of the retinal dysplasias, especially the inherited ones. We will use the following scheme for now as though its “gospel,” but just keep in the back of your mind that it’s an oversimplification:
Multifocal - Inherited in many breeds (most notably the Cocker Spaniel).
Geographic (also called generalized) - English Springer Spaniel, Labrador
Multifocal Retinal Dysplasia
Clinical signs/history
- None. An incidental finding on routine retinal exam; some breeders request a retinal exam so they can discover this problem in their breeding animals.
Ophthalmoscopic / histologic appearance
Histologically, retina is thrown into folds, or photoreceptors are disorganized into “rosettes.”
Linear, dot shaped, or vermiform streaks in tapetum or nontapetum.
In tapetum, usually green or gray lesions, sometimes surrounded by hyperreflectivity
In nontapetum, usually white lesions.
Can see identical fold lesions in some animals that are not due to abnormal retinal differentiation, but rather due to different growth rates of the neurosensory retina and the remainder of the posterior pole of the eye – these disappear as animal grows (most commonly seen in Collies).
Etiology
- Inherited, autosomal recessive
Treatment
- No treatment available or needed. These lesions are nonprogressive and do not cause noticeable visual impairment.
Geographic Retinal Dysplasia
Clinical Signs/history
- Depends on severity; usually some degree of visual impairment, sometimes completely blind. Occasionally no noticeable visual impairment.
Ophthalmoscopic / histologic appearance
Histology - Large coalescing areas of folds, rosettes
Ophthalmoscopy - Large pigment clumps in tapetum, usually immediately superior to the optic disc. Clumps are usually surrounded by hyperreflectivity. In the more severly affected animals, the retina may be detached.
Etiologies
English Springer - Inherited; autosomal recessive. Will sometimes see multifocal lesions in Springers as well. Because relationship of these lesions to the geographic lesions is unknown, it is recommended to assume all Springer dysplasias are serious and warrant not breeding.
Labrador Retriever - Inherited. Skeletal dwarfism may accompany this lesion. Prevalent thought is that these lesions are caused by a single gene that has recessive effects on the skeleton, incomplete dominant on the retina. Homozygotes are dwarfed, have the most severe ocular lesions, heterozygotes have milder ocular lesions (sometimes just multifocal), and no skeletal lesions.
Treatment
- None. Even though there is debate about the genetics in these breeds and whether or not multifocal syndromes unrelated to the geographic syndromes exist, it is currently recommended that they not be bred regardless of the severity of the dysplasia in these breeds.
# Canine Multifocal Retinopathy
Definition
- An unusual early onset (10-12 weeks) retinopathy manifesting as focal to multifocal serous retinal detachments
Breeds: Pyrenees, Coton du Tulear, Mastiff
Clinical signs/history
None. An incidental finding on routine retinal exam; some breeders request a retinal exam so they can discover this problem in their breeding animals.
Ophthalmoscopic appearance
Multifocal gray to tan fundic patches varying in size from pinpoint to about the size of the optic disc
Lesions often near the optic disc and around major veins
Etiology
- Inherited, autosomal recessive. Mutation in the BEST1 gene
Treatment
- No treatment available or needed. These lesions are nonprogressive and do not cause noticeable visual impairment
# Inherited Retinal Degenerations
This set of retinal diseases is characterized by retinas that develop in pretty good shape, at least up until the time of birth. This may seem an odd statement at first….after all, isn’t the body pretty much developed at birth, with only growth and some “fine tuning” and refinement occurring thereafter? Turns out that the canine retina is not fully developed at birth……in fact, this is true of many species. The pieces and parts are pretty much all there, but histologically and functionally the retina is not that of a mature dog until 7-8 weeks of age. This can be appreciated from the fact that the ERG is not “mature” until that time, the menace response is often absent until that time, and even the appearance of the retina changes during that time (e.g., the tapetum, if the dog is destined to have one, goes from a dull gray color, to a bluish violet (similar to Elizabeth Taylor’s iris color) to whatever it’s adult coloration is going to be (generally a yellow or green) over about an 8 week period).
The first two categories of Inherited Retinal Degenerations (Early Onset Photoreceptor Degenerations and Late Onset Photoreceptor Degenerations) are collectively called Progressive Retinal Atrophy (PRA). PRA is usually spoken of as if it were a single disease, with a given etiology, set of clinical signs, clinical progression, ophthalmoscopic appearance, treatment (spoiler alert: there isn’t one!), and prognosis. But PRA is probably more appropriately discussed as a group of diseases, the PRAs, because the underlying genetic mutations and resulting cellular pathologies can be very different from breed to breed. So below we will start with a discussion of PRA as a single disease entity (highlighting the similarities), then we will give a brief overview of the individual PRAs that represent both early onset photoreceptor degenerations and late onset photoreceptor degenerations.
A word of warning: as the PRAs have been discovered over the past 100+ years, there has not been a consistency in the way they were named….some were named after what was occurring histologically (e.g., rod-cone dysplasia in Setters, Collies and Corgis……because while the retina in general develops normally up until the time of birth, further postnatal development is dysplastic), while others have more of a clinical description (e.g., early retinal degeneration in Elkhounds….because the retina apparently develops normally in the early postnatal months, but structure and function fade shortly thereafter). Sorry about that…if it were left up to me, I’d have called them PRA1, PRA2, PRA3, etc., but nobody asked me!
# General Appearance of the Degenerated Retina:
Hyperreflectivity of tapetum
Remember that the tapetum is a choroidal structure, not a retinal structure
Obviously the tapetum is inherently reflective
With retinal degeneration, the retina overlying the tapetum is thinned, resulting in less attenuation of light shined onto the fundus and less attenuation of the reflection coming back from the tapetum
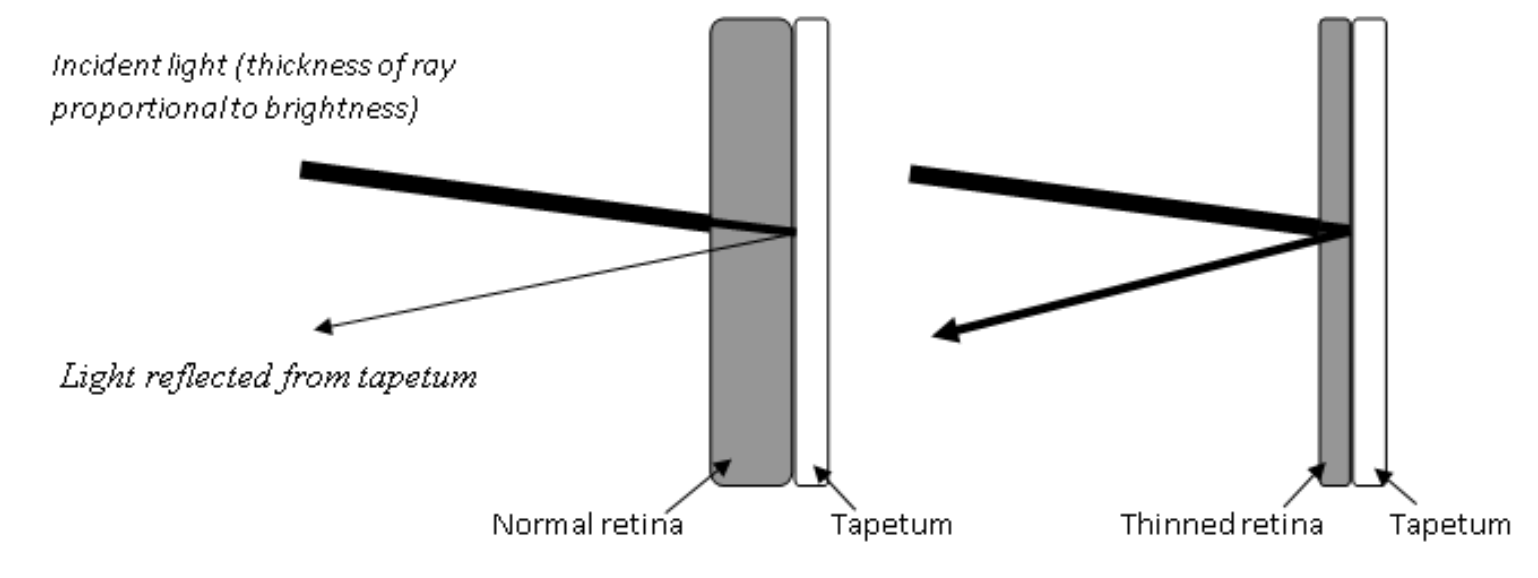
Hyperreflective lesions can be widespread or focal/multifocal, depending on the specific degenerative disorder
Vascular attenuation
As the retina degenerates, its need for oxygen is reduced so the vasculature can waste away.
If the degenerative disorder is focal/multifocal, you generally won’t appreciate vascular attenuation. Vascular attenuation is more of an issue with diffuse degenerative disorders.
Depigmentation of the nontapetum with pigment clumping
- Also appreciated more with widespread degenerative disorders.
Gray radial streaking of the tapetum
Optic nerve atrophy in the late stages
# Suspected Inherited Retinal Degenerative Disorders
# Progressive Retinal Atrophy
What all (well, almost all) PRAs have in common.
Definition: A group of inherited, acquired photoreceptor diseases characterized by slowly progressive bilateral vision loss. Almost all classes of PRA are due to loss of rod cell function first with subsequent progression to loss of cones and complete blindness.
Clinical signs/history:
Initial clinical sign is almost always nyctalopia
Owner may notice increased “eyeshine” due to pupillary dilation and increased reflectivity to the tapetum.
Blindness progresses and generally becomes complete….i.e., blind in all lighting conditions
Cataract formation is common
- Not entirely sure why….some say it’s due to release of cataractogenic substances during the retinal degenerative process
PLRs may be normal with early disease, sluggish with midstage disease and absent with end stage disease (but variations occur).
Ophthalmoscopic appearance:
Changes as the disease progresses
Diffuse tapetal hyperreflectivity due to retinal thinning
Attenuation of retinal vasculature
Grayish radial streaks in the tapetum
Diffuse depigmentation with pigment clumping in the nontapetum
Eventual atrophy of the optic nerve
Etiology:
Inherited; almost always autosomal recessive.
Note that several different genetic mutations have been identified. See the individual gene abnormalities below
Diagnosis
Most are treated on the basis of consistent history together with ophthalmoscopic appearance outlined above. Note that for some breeds (details below) the disease might not become phenotypically affected until several years old.
ERG – ERGs are abnormal long before dog becomes phenotypically affected.
Molecular genetic testing – There are genetic tests available for many of the PRAs. The advantage of this method is that the dogs can be diagnosed as soon as you can collect a buccal swab, and carriers as well as dogs destined to be phenotypically affected can be identified (ophthalmoscopy and ERG can only identify phenotypically affected animals).
Treatment
- None. Counsel owner as to maintenance of a blind animal. Dogs with PRA should not be bred.
# Early Onset Photoreceptor Degenerations
PRAs in which postnatal retinal development goes awry resulting photoreceptor dysfunction
Rod-cone dysplasia 1 (RCD1)
Breed(s) – Irish Setter
Age of onset – nyctalopia at 6-8 weeks, complete blindness by 1 yr
Defective gene – PDE6B
Result of gene defect - β-subunit of calmodulin dependent cGMP phosphodiesterase is abnormal, resulting in elevated cGMP levels in the retina
Retinal appearance – typical PRA retina
ERG – completely extinguished by 18 weeks
RCD2
Breed(s) – Collie
Age of onset – nyctalopia at 6-8 weeks, complete blindness by 1 yr
Defective gene – rd3
Result of gene defect - β-subunit of calmodulin independent cGMP phosphodiesterase is abnormal, resulting in elevated cGMP levels in the retina
Retinal appearance – typical PRA retina
ERG – completely extinguished by 18 weeks
RCD3
Breed(s) – Cardigan Welsh Corgi
Age of onset – nyctalopia at 6-8 weeks, complete blindness as a “young adult”
Defective gene – PDE6A
Result of gene defect - α-subunit of independent cGMP phosphodiesterase is abnormal, resulting in elevated cGMP levels in the retina
Retinal appearance – typical PRA retina
ERG – completely extinguished very young, but exact age unspecified
Early Retinal Degeneration
Breed(s) – Norwegian Elkhound
Age of onset – nyctalopia at 6-8 weeks, complete blindness by 12-18 mos
Defective gene – STK38L
Result of gene defect – abnormal neural transmission in outer plexiform layer of retina
Retinal appearance – typical PRA retina
ERG – completely extinguished very young, but exact age unspecified
Photoreceptor Dysplasia
Breed(s) – Miniature Schnauzer
Age of onset – histologic and ERG abnormalities apparent by a few months of age, but clinical progression is a bit more delayed compared to the RCDs and Early Retinal Degeneration
Defective gene – type a-PRA
Result of gene defect - unknown
Retinal appearance – typical PRA retina, but sometimes hard to apprectiate because Schnauzers have a lot of variation in the normal tapetal appearance.
ERG – completely extinguished very young, but exact age unspecified
Cone-rod dystrophies (CRD1, CRD2, CRD3) – Unique among PRAs in that the cones and rods are pretty much equally affected, with cones affected earlier.
Breed(s) – Variations in the Staffordshire, Pit Bull and Glen of Imaal Terrier, but we’ll concentrate on the disease in Short haired Dachsunds
Age of onset – histologic and ERG abnormalities apparent by a few months of age, but clinical progression is a bit more delayed compared to the RCDs and Early Retinal Degeneration
Defective gene – NPHP4
Result of gene defect - unknown
Retinal appearance – typical PRA retina
ERG – cone component gone by 5 weeks of age; rod component a bit later.
# Cone Degeneration
Definition: A recessively inherited disease of cone cells in affected Alaskan Malamutes and German Shorthaired Pointers that causes day blindness.
Clinical signs/history:
Day blindness….i.e., vision loss exclusively in bright daylight or high levels of artificial illumination
Recovery of vision when returned to lower levels of illumination takes several minutes
Ophthalmoscopic appearance:
- None! Retinas look completely normal.
Etiology:
Inherited; autosomal recessive.
Defective gene: CNGB3
Diagnosis
Appropriate combination of breed, observation of day blindness with normal vision in dim light, normal retinal exam, and characteristic ERG findings
ERG – rod cell ERG responses are normal, but cone cell ERG is extinguished.
Treatment
- None.
# Retinal Pigment Epithelial Dystrophy
Definition: A group of conditions with ophthalmoscopic changes that can be characterized by an accumulation of irregular foci or light-brown pigment spots in the central tapetal fundus. Over time, these foci increase in size and become distributed throughout the tapetal zone. Initially these dogs lose central visual acuity which can be very subtle. Progression is very slow, so many owners never really appreciate vision loss. The clinical and ophthalmoscopic features of this disorder are almost identical retinal disease associated with vitamin E deficiency. Your text uses the term “central progressive retinal atrophy” (CPRA) which is not a good term for the syndrome because it is in no way associated with PRA outlined above.
Clinical signs/history:
- Often none. This disorder is most often diagnosed on screening exams in which the owners are specifically investigating for it. Because it occurs in hunting breeds, astute owners may notice behaviors that can be attributed to a loss of central visual acuity
Ophthalmoscopic appearance:
- Focal pigment accumulations in the tapetal fundus that eventually develop hyperreflectivity around them.
Etiology:
Inherited, but with mysterious genetics. Thought to be dominant in the Labrador, Chesapeake and Border Collie, but recessive in the Briard.
Obviously this condition represents different underlying disorders that have a similar ophthalmoscopic appearance
Diagnosis
Ophthalmoscopy
ERG – normal
Treatment
- None.
# Nonherited Retinal Degenerations
# Sudden Acquired Retinal Degeneration Syndrome (SARDS)
Definition: An acquired disorder of retinal function characterized by sudden onset of complete blindness with initially normally appearing retinas. The basic pathophysiology is photoreceptor destruction of unknown cause.
Clinical signs/history:
Sudden onset complete blindness (hours to days)
Middle aged, overweight females of small breeds (esp Schnauzer and Dachsund) are over-represented.
PLRs are usually maintained, although they may be slow and incomplete
No difference in dim vs. bright light
Often PU/PD, polyphagic
Ophthalmoscopic appearance:
Initially normal: the old adage is “the dog sees nothing, and the vet sees nothing!”
With time, typical signs of retinal degeneration appear (i.e., tapetal hyperreflectivity and vascular attenuation), but they almost never reach the degree of ophthalmoscopic abnormality as seen in PRA dogs. These retinal changes usually don’t become apparent until the dog has been blind for many months.
Etiology:
Unknown
One theory in the past has been excitotoxicity….i.e., toxicity due to excitatory neurotransmitters. What is unclear, however, is whether the elevated levels of excitatory neurotransmitters is cause or effect.
Because this disorder resembles an immune-mediated retinopathy in humans called cancer-associated retinopathy, some ophthalmologists feel this may be an immune-mediated disease.
The association with other endocrinopathies (as suggested by the pu/pd/polyphagia) is unknown, but the most recent publications suggest that a higher percentage of these dogs are Cushingoid than previously believed.
Diagnosis
Combination of history of sudden onset blindness with an otherwise normal eye exam….i.e., you’ve ruled out glaucoma, retinal detachment, etc.
ERG will be extinguished
Recently chromatic pupillography has been shown to be highly diagnostic for SARDS
Since this is a photoreceptor disease, and photoreceptor activation is the first step in the PLR, one would expect the PLRs to be absent….but as mentioned above, this is not what we usually witness, as PLRs are usually present but somewhat sluggish
A few years ago a group of researchers discovered that a small subset of ganglion cells (about 2% of them) can also respond to photons of light……i.e., they can also be “photoreceptors.”
Recall that the photopigment in rod cells is rhodopsin. The photopigment in these ganglion cells is “melanopsin.”
Turns out the axons from melanopsin-containing ganglion cells all either subserve PLRs (or circadian rythyms)….thus some PLR can remain because it is elicited by this 2% of melanopsin-containing ganglion cells. But none of these cells is connected to the visual cortex, so no vision is retained.
Also turns out that melanopsin has a strong preference for short wavelengths of light……it binds photons of bluish wavelenths of light much more avidly than photons of reddish wavelengths. As a result, white light PLRs (which contain all wavelengths) are present but sluggish, blue light specific PLRs are essentially normal, and red light PLRs are essentially absent. Thus, the term “chromatic pupillography.”
Treatment - None. Counsel owner as to maintenance of a blind animal.
# Vitamin E deficiency retinopathy
Vit E is an antioxidant that helps maintain cell membrane stability by preventing lipid peroxidation.
Dietary deficiency of vit E (or inherited vit E absorption or metabolism anomalies) causes retinopathy indistinguishable from RPE dystrophy (see above).
Also causes CNS, repro and muscle pathology
Seems to be seen mostly in hunting dogs
# Glaucomatous Retinal Degeneration
As mentioned in the glaucoma lectures, the primary pathology of glaucoma is destruction of the retinal ganglion cells (RGCs), and they ARE a part of the retina….so by definition glaucoma does cause retinal degenerative disease.
With time elevated IOP also causes damage to other retinal cell types.
Glaucomatous retinal degeneration tends to occur in wedge-shaped areas with the apex at the optic nerve….so-called “watershed lesions.”
# Post-inflammatory Retinal Degeneration
Definition of chorioretinitis – Inflammation of the retina and choroid. Because these structures are in such intimate contact anatomically and pathophysiologically, we almost never see inflammation of just the choroid (choroiditis) or just the retina (retinitis).
This topic is being introduced here because the ophthalmoscopic appearance of post-inflammatory retinal lesions has similarities to the ophthalmoscopic appearance of all other retinal degenerative diseases. Active chorioretinal inflammation looks different, and we’ll cover that later.
We will discuss the etiologies, treatments, etc. of chorioretinitis in detail later in a later section. Right now we just need to mention the appearance of the chorioretinal scars that result from chorioretinitis since they are a form of retinal degeneration.
Ophthalmoscopic appearance of chorioretinal scars:
Usually focal / multifocal lesions
Hyperreflective
Generally don’t appreciate vascular attenuation since lesions are so small
Well-demarcated
Flat
Not associated with hemorrhage or cellular exudation
Sometimes have clump of pigment in center of lesion (“cigarette burn” lesions)
Significance of chorioretinal scars
Usually none; just an incidental finding
Can cause vision impairment if widespread
# Retinal Inflammatory Diseases
# Chorioretinitis
Definition: Inflammation of the choroid and retina. Almost never have inflammation of these 2 structures independently, and even if it does occur is indistinguishable ophthalmoscopically.
Clinical signs/history
- Because chorioretinitis is usually nonpainful and because the structures in question are in the back of the eye, owner will usually not be aware of any problems. The exception to this is if the lesion(s) are very widespread and/or have resulted in retinal detachment, in which case the animal will be blind in the affected eye. Also, chorioretinitis is frequently accompanied by anterior uveitis, which means that all of the clinical signs of uveitis (blepharospasm, photophobia, etc.) may be present. Also, like uveitis, chorioretinitis is frequently a manifestation of systemic disease, which means that the animal may have clinical signs/history referable to such diseases (e.g., blasto dog may be listless, have a cough, etc.).
Ophthalmoscopic appearance
Active lesions
Focal / multifocal
Dull gray, white in color
Poorly demarcated
May have “mass”
May have retinal hemorrhage
Sometimes retinal vessels engorged
Inactive, quiescent lesions
Focal / multifocal
Hyperreflective if in tapetum; depigmented if in nontapetum
Well demarcated
Flat
Not associated with hemorrhage
May have pigment clump in middle of lesion
Etiologies
Infectious - Systemic fungal, rickettsial, viral (CDV), parasitic (toxocara), protozoal (toxoplasmosis), etc.
Immune-mediated - VKH
Cardiovascular - Hypertension
Neoplastic - Multiple myeloma, lymphosarcoma
Treatment:
- Usually no specific treatment for the retina. Treat underlying systemic disorder.
Outcome:
- Assuming retinal lesions resolve with treatment of underlying disorder, retinal function in the affected area may or may not return. The retina can’t take much damage. If inflammatory lesions are of any severity and persist for as little as a few days, you can expect the affected area to be nonfunctional. Fortunately, inflammatory lesions usually occupy a relatively small portion of the retinal surface area, so functional vision is usually unaffected.
# Retinal Vascular Disorders
# Systemic Hypertension
Does occur in dogs, but is much more common in cats
Generally secondary; most common causes:
Hyperadrenocorticism
Chronic renal disease
Phaeochromocytoma
Ophthalmoscopic findings
Retinal hemorrhage
Bullous retinal detachment (usually focal or multifocal but can be complete)
Rarely, hyphema and/or anterior chamber fibrin
Diagnosis
Significantly elevated systolic BP (>170 mmHg; usually >200 mmHg)
Moderate elevations (in the 140-170 mmHg range) rarely cause ocular signs
Treatment
Treat the underlying hypertension
- ACE inhibitors, β-blockers, Ca2+ channel blockers, etc.
No specific therapy for the retinal lesions; they should resolve with successful therapy of the hypertension, although chorioretinal scars will probably form where hemorrhages and detachments were.
# Hyperviscosity Syndrome
Retinal lesions can occur with any condition that drastically raises serum viscosity
Causes:
Multiple myeloma
- Viscosity rises due to hyperglobulinemia associated with monoclonal gammopathy
Erlichiosis
- Viscosity rises due to hyperglobulinemia associated with polyclonal gammopathy
Ophthalmoscopic findings
Dilated tortuous retinal vessels
Retinal hemorrhage
Bullous retinal detachment (usually focal or multifocal but can be complete)
Rarely, hyphema and/or anterior chamber fibrin
Diagnosis
Combination of appropriate retinal signs together with documentation of a disorder associated with hyperviscosity
Serum viscosity can be measured in the clin path lab
Treatment
Treat the underlying disorder
No specific therapy for the retinal lesions; they should resolve with successful reduction of serum viscosity, although chorioretinal scars will probably form where hemorrhages and detachments were.
# Retinal detachment
Definition/Histology
- Separation of the neurosensory retina from the underlying retinal pigment epithelium. Generally results in marked vision loss, and sometimes complete blindness. Usually a peracute presentation.
Types of Detachment
Serous (also called bullous)
- Separation due to accumulation of inflammatory material, transudate, hemorrhage, etc. between retina and RPE.
Rhegmatogenous
- Separation begins as a tear, usually in the peripheral retina. Detachment then progresses as vitreal fluid accumulates between the RPE and the neurosensory retina.
Ophthalmoscopic appearance
- Anterior ballooning of the retina. Since the retina is translucent, may be barely able to distinguish the retina itself – use retinal vessels as a clue. In addition, in serous detachments the subretinal fluid is generally very hazy, murky, or bloody.
Etiologies
Serous / Bullous:
Manifestation of chorioretinitis
Manifestation of retinal dysplasia or CEA
Steroid-responsive retinal detachment - Syndrome of sudden onset bullous detachment (usually bilateral); sudden blindness; suspected immune- mediated. German Shepherds predisposed.
Rhegmatogenous:
Post cataract surgery, especially if lens was hypermature.
Spontaneous
- Seen most commonly in the Shih Tzu and Lhasa Apso
Clinical signs
- Blindness. Signs referable to systemic disease if in association with systemic disease.
Treatment
Serous / bullous detachment
If associated with chorioretinitis - Treat underlying disorder
If associated with retinal dysplasia or CEA - No treatment (but don’t breed)
Steroid responsive detachment - Immunosuppressive doses of corticosteroids.
Prognosis depends on how long the retina has been detached.
Rhegmatogenous
Retinal reattachment surgery
Really cool
Not many ophthalmologists offer this procedure (maybe 5 or 6 in the U.S.?)
Roughly 90% successful at getting retina to remain attached post op. Roughly 80% have a return to vision, and the sooner surgery is performed the better the prognosis
# OPTIC NERVE DISORDERS
Remember that the optic nerve is not truly a cranial nerve, but a white matter tract of the diencephalon ***composed principally of the axons of retinal ganglion cells (RGCs)***. RGC axons project without synapses from the retinal nerve fiber layer (NFL) through the optic chiasm and the optic tracts to either the lateral geniculate nucleus (LGN), superior colliculus, hypothalamus, or pretectal nucleus and other midbrain centers.
The optic nerve consists of four different regions. The intraocular (or “intrabulbar”) optic nerve includes the RGC layer, NFL, ONH or optic disc, and the intralaminar (i.e., the portion that passes through the lamina cribrosa) optic nerve region within the sclera. Posterior to the globe, the optic nerve consists of the intraorbital (aka “retrobulbar”) optic nerve, the intracanalicular optic nerve (within the optic canal of the skull), and the short intracranial optic nerve that merges into the optic chiasm. The optic nerves form the optic tracts between the optic chiasm and the LGN, and the optic radiations between the LGN and visual cortex.
# Optic Neuritis
Definition: Inflammation of the optic nerve.
Intrabulbar - The optic nerve within the eyeball itself (i.e., the optic disc) is inflamed.
Retrobulbar - Only the portion behind the globe is inflamed.
Clinical signs/history:
Sudden onset blindness (hours to a week or so).
Pupils dilated and nonresponsive….compare to SARDS
Ophthalmoscopic appearance
Intrabulbar - Swollen, edematous, hyperemic optic disc. The swelling and edema are termed “papilledema” (see below). Disc margins very fuzzy and indistinct. Disc sometimes hemorrhagic. Sometimes have peripapillary edema of the retina, which appears as a fuzzy ring around the disc.
Retrobulbar - Fundus appears completely normal. DOG SEES NOTHING, VETERINARIAN SEES NOTHING!
Etiologies:
Infectious - CDV, toxoplasmosis, systemic fungal (esp. crypto)
Immune mediated – Granulomatous meningoencephalomyelitis (GME)
Neoplastic - Lymphoma
Idiopathic - Account for about 90% of optic neuritis cases
Diagnosis:
Ophthalmoscopy (if intrabulbar)
Clinical history and lack of ophthalmoscopic signs (if retrobulbar); retrobulbar differentiated from SARDS via ERG and VEP or response to therapy.
Also use PLRs to differentiate from SARDS
CSF tap may help establish etiology (cytology, culture, CDV or toxo titers).
Treatment:
If an infectious or neoplastic etiology is established, treat it.
Otherwise immunosuppressive doses of corticosteroids – usually responds rapidly to treatment.
GME also a steroid responsive condition, but usually relapse and eventually die.
Sequelae:
- Tends to be recurring disease in some instances. Steroids may be needed for long periods, and animal may need repeated bouts of steroids. With each recurrent attack, a little more irreversible damage is done, so these animals frequently end up with optic disc atrophy (small, gray disc).
# Optic Nerve Hypoplasia
Definition: Small optic nerve in which the number of RGC axons is so low that vision is reduced or absent and PLRs are abnormal.
Reported in many breeds, but I tend to associate it primarily with Poodles and Shih Tzus.
Can be unilateral or bilateral
Can be a difficult diagnosis because of the variation in size among normal optic nerves. Some dogs have exceptionally small nerves but retain apparently normal vision and PLRs. This condition may be called “micropapilla” and is seen most commonly in the Belgian Sheepdog and Belgian Tervuren.
Clinical signs
Unilateral or bilateral blindness
May be found incidentally (i.e., unbeknownst to the owner) if unilateral
Ophthalmoscopic appearance
- Small optic nerve
Etiology
Usually uknown
Many feel there’s a genetic basis, but reports on heritability are confusing
Diagnosis
- Ophthalmoscopy
Treatment
- None
# Papilledema
Definition: Swelling of the optic disc
- May occur alone or as one of the manifestations of optic neuritis (see above)
Clinical signs
If just papilledema, none
If swollen due to optic neuritis, eye will be blind
Ophthalmoscopic appearance
Swollen optic nerve
Can tell that the nerve is projecting anteriorly into the vitreous because vessels will change direction as they cross the edge of the disc
If part of optic neuritis, will also be hyperemic, hemorrhagic, fuzzy margins, etc (see optic neuritis above)
Etiology
If just papilledema without neuritis, is due to anything that retards cerebrospinal fluid flow
Most commonly hydrocephalus
Optic nerve tumors
If papilledema as part of optic neuritis: see etiologies of optic neuritis above
Diagnosis
- Ophthalmoscopy
Treatment
- Treat underlying condition